Commitment
Our commitment to business development remains unwavering. We have explored uncharted territories, forge partnerships that drive innovation, practiced dedication for a decade to quality & compliance, contributed to a healthier and more prosperous future for all and has earned a reputation as a trusted partner in the healthcare industry.With a steadfast commitment to excellence, Avian International has been at the forefront of medical device and pharmaceutical exports, delivering innovative solutions that make a tangible impact.
Approach
Our approach to business development is built on a foundation of Expertise, collaboration, and a resolute dedication to shaping the future of healthcare. By synergizing our profound market knowledge with the expertise of our esteemed partners in Research & Development (R&D) and Manufacturing, we pave the way for cutting-edge solutions that transcend boundaries. Our dynamic approach to business development fuels innovation and fosters collaborations that elevate the standards of medical devices and pharmaceuticals worldwide. At Avian, we believe in creating shared success.
Using our extensive network across diverse markets, we recognize the demand for products and strive to introduce them by identifying suitable companies capable of developing and manufacturing products according to the market's requirements.
Fostering Innovation through Partnerships
Avian acts as a partner in product development at different stages of the development process. We also explore the market in person to discover new products for various therapeutic categories that are currently unavailable. These products have the potential to improve patient well-being and contribute positively to the country's healthcare landscape. With our skilled team of experts, we collaborate closely with manufacturing partners to develop these new products.
In a highly regulated industry, compliance is non-negotiable. Our business development strategy places immense importance on adhering to global standards and regulations.
We take initiates to get approvals from various regulatory bodies across the globe and trigger plant audits.
We understand regulatory requirements of various countries
We organize preauidts for our manufacturing partners
We take responsibility to solve the query for product registrations by actively participating in regulatory processes. From organizing a pre audit, keeping track of non conformities, reaching out to generating corrective actions and applying it to systems.
Ultimately, our mission is grounded in making a positive impact on patient lives. Our medical devices are designed with patients in mind, aiming to improve their quality of life, enhance treatment outcomes, and empower healthcare providers with effective tools.
One of our business development strategies is a patient-centric ethos. Our dedicated field force maintains continuous engagement with patients in the market. Once our products are integrated into the treatment plans by healthcare professionals, we take immense pride in ascertaining their satisfaction with the outcomes. Their allegiance to our products is a testament to their efficacy, as they faithfully adhere to usage recommendations. This enduring commitment underscores the recurrent utilization of our offerings, validated by their continued usage in alignment with prescribed regimens. This not only underscores our unwavering dedication to patient well-being but also showcases the sustainable growth trajectory of our enterprise.
We assist our local partners in every country, going beyond product distribution to actively promoting them among end users such as doctors, pharmacies, and hospitals. This distinct approach sets us apart and provides a competitive edge by fostering robust relationships with our import partners. By participating in the product sales within their respective countries, we contribute to our import partners' success, resulting in mutual benefits.
Our approach to market engagement is multifaceted, centered around fostering on-the-ground exchange of product knowledge. We understand that effective marketing strategies require more than just product distribution. Hence, we actively participate in Continuing Medical Education (CME) events for doctors and paramedical staff, ensuring they are well-informed about our offerings.
Furthermore, we are committed to empowering our local partners by providing comprehensive training, equipping them with the skills and knowledge needed to represent our products effectively. To bolster their efforts, we offer promotional materials that communicate the value and benefits of our products.
Being an integral part of product launches allows us to create a buzz in the market and generate excitement among potential users. By combining these strategies, we establish a strong presence in each market, ensuring that our products not only reach end-users but also meet their needs effectively. Our commitment to knowledge exchange, training, and marketing innovation strengthens our partnerships and drives success for all stakeholders involved.
Our Flexible Production Method allows us to respond swiftly to changes, ensuring that you receive the medical devices you need precisely when you need them. This agility empowers you to better serve your patients and stay ahead of industry trends. Our production approach allows us to offer customization options, ensuring that the medical devices you receive align perfectly with your patient care strategies. Your input drives our design, resulting in products that truly meet your unique requirements.
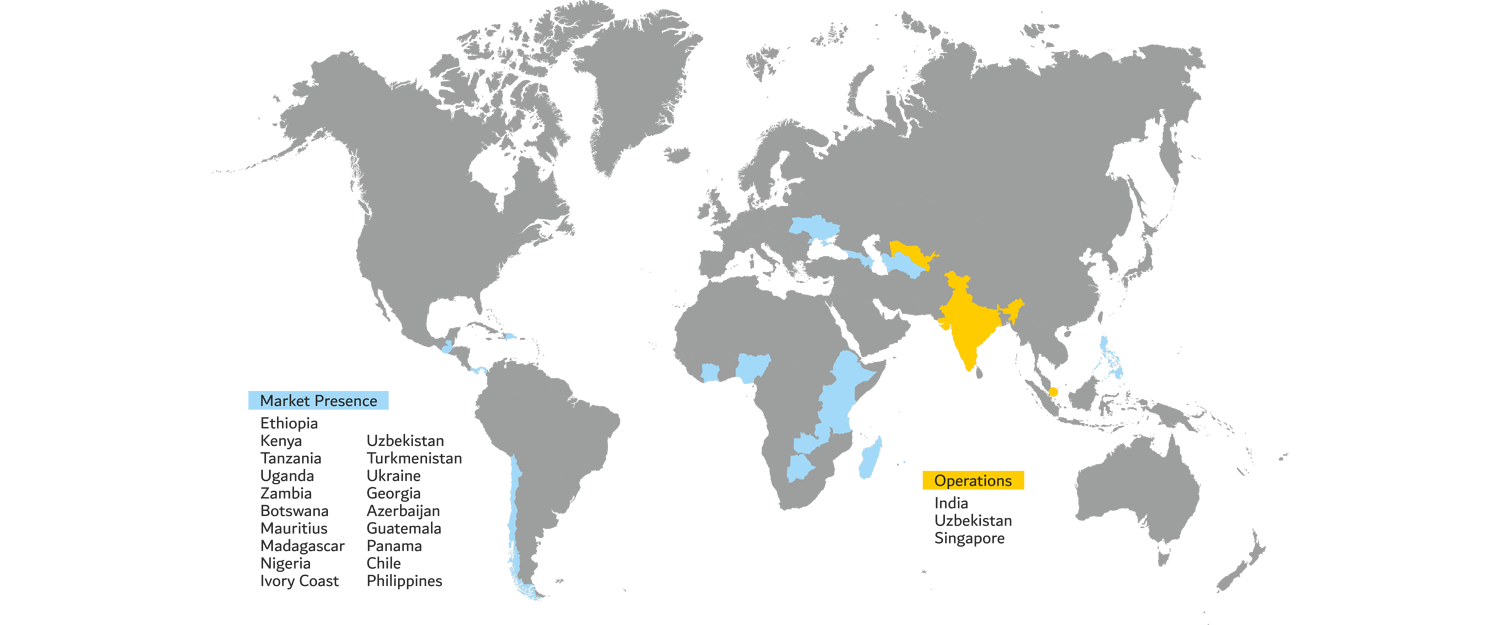
Exporting Excellence Worldwide
Avian International's impact extends across the globe. With a robust distribution network, strategic partnerships, and local representatives, we have cultivated a global reach that enables us to bring life-changing healthcare solutions to communities far and wide. Our dedication to meeting international standards ensures that our products are accessible, reliable, and trusted in markets around the world.